The Ministry of Public Health (MoPH) has dismissed allegations that a six-year-old child died from ingesting drugs developed to fight off the filaria virus.
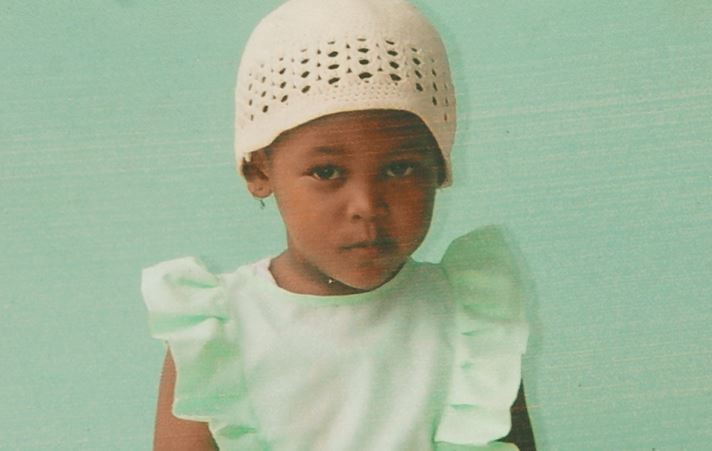
Princess Kissoon of D Field Sophia, Georgetown died on October 27, two days after she consumed half of one of the filaria pills being distributed on a mass countrywide campaign against the disease.

The Ministry in a statement Tuesday extended its sincerest condolence to the family of the late Kissoon who passed away at Georgetown Public Hospital.
However, the Ministry contended that the drugs could not have caused the child’s death.
“The Post Mortem examination performed by Pathologist Dr. Nancy Olario on October 29 does not point to any linkage between the ingestion of the tablet and the Kissoon child’s death,” the statement read.
The autopsy, according to the child’s father Ray Kissoon, said the cause of death was inconclusive and the family is trying to raise funds to conduct a second autopsy at a private institution.
Hpwever, the Ministry in its statement asserted that the drugs being administered are safe.
Little Princess had suffered a high fever two days after she ingested the drug and she died shortly after her father rushed her to the Hospital.
Her family is blaming the public health workers for her death, contending that the pills may have negatively affected the young child.
The family called for health workers involved in the campaign to be better trained to guide families on the usage and side effects of the treatment.
Meanwhile, the Ministry explained that the Global Programme to Eliminate Lymphatic Filariasis began in 2001 in Guyana.
Since the year 2008, six regions have benefitted from the Mass Distribution of the tablets Diethylcarbamazine (DEC) 100mg and Albendazole 400mg at predefined doses.
In 2016, 277,612 persons from Regions 3, 4, 5 and 10 received the drugs via directly observed therapy (DOT). Prior to this initiative, thousands of Guyanese received Diethylcarbamazine in DEC salt.
The medicines being distributed are donations from the World Health Organization (WHO) through their regional office – the Pan American Health Organization.
The Ministry explained that the donation process begins with the exchange of documents which include a certificate of good manufacturing practice (issued by the Ministry of Health in the country where the tablets are manufactured), a certificate of analysis of the tablet which assures product quality assurance and an information leaflet on the tablet which details its indications, considerations and precautions, dosage and side effects.
The Ministry explained that the documents are first perused by the Permanent Secretary and Chief Medical Officer of the Ministry of Public Health before the drugs are approved for shipment.
The Ministry further noted that the adverse effects of the drugs are uncommon and occur mostly in patients who have a significant worm burden.
Adverse effects subside within 24 hours and any person who experiences these adverse effects beyond this time should seek medical attention, the Ministry stated.
It boasted that in the nine years of tablet distribution in the country, there have been no serious adverse events that have led to hospitalization, disability or death.
The Ministry said it will continue to provide the treatment in an ongoing effort to protect the public from the chronic, debilitation caused by filariasis.