Gov’t recalls three baby formulas following infections, death in the US
The Government Analyst Food and Drug Department has recalled three baby formulas following three complaints of infections and one death in the United States after the infants reportedly consumed the products.
Persons are being urged to avoid Similac, Alimentum and EleCare powdered baby formulas. These specific types of formulas were produced by Abbott Laboratories in Michigan, United States and an investigation has been launched by the US Food and Drug Department to determine whether the infections are linked to the lab.
The government here is now issuing a warning to the public to avoid purchasing or discontinue use of these formulas and, where possible, return to the point of purchase.
A statement by the Food and Drug Department noted that the local distributor has also initiated a recall exercise.
“The department received an official communication from the manufacturer indicating that a proactive, voluntary recall has been initiated due to four consumer complaints in the United States related to Cronobacter sakazakii or Salmonella Newport in infants who have consumed the infant formula manufactured in Abbott Nutrition’s Sturgis, Michigan facility,” the Food and Drug Department said in its statement on Tuesday.
Meanwhile, it must be noted that Cronobacter bacteria can cause severe, life-threatening infections such as sepsis or meningitis.
Symptoms of sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice, grunting breaths and abnormal movements. Cronobacter infection may also cause bowel damage and may spread through the blood to other parts of the body.
Symptoms of Salmonella bacteria include diarrhea, fever, and abdominal cramps.
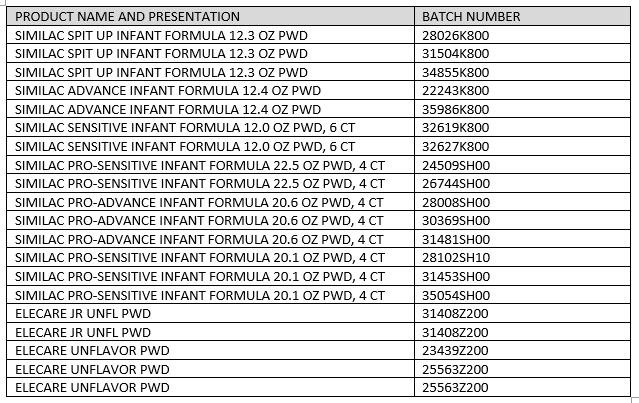
The implicated powdered infant formula products are:
Further information concerning the public warning issued could be found on the US FDA website, here: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/abbott-voluntarily-recalls-powder-formulas-manufactured-one-plant.
In addition, the department can be contacted at 222- 8859/60 for further guidance relating to this public health announcement.






